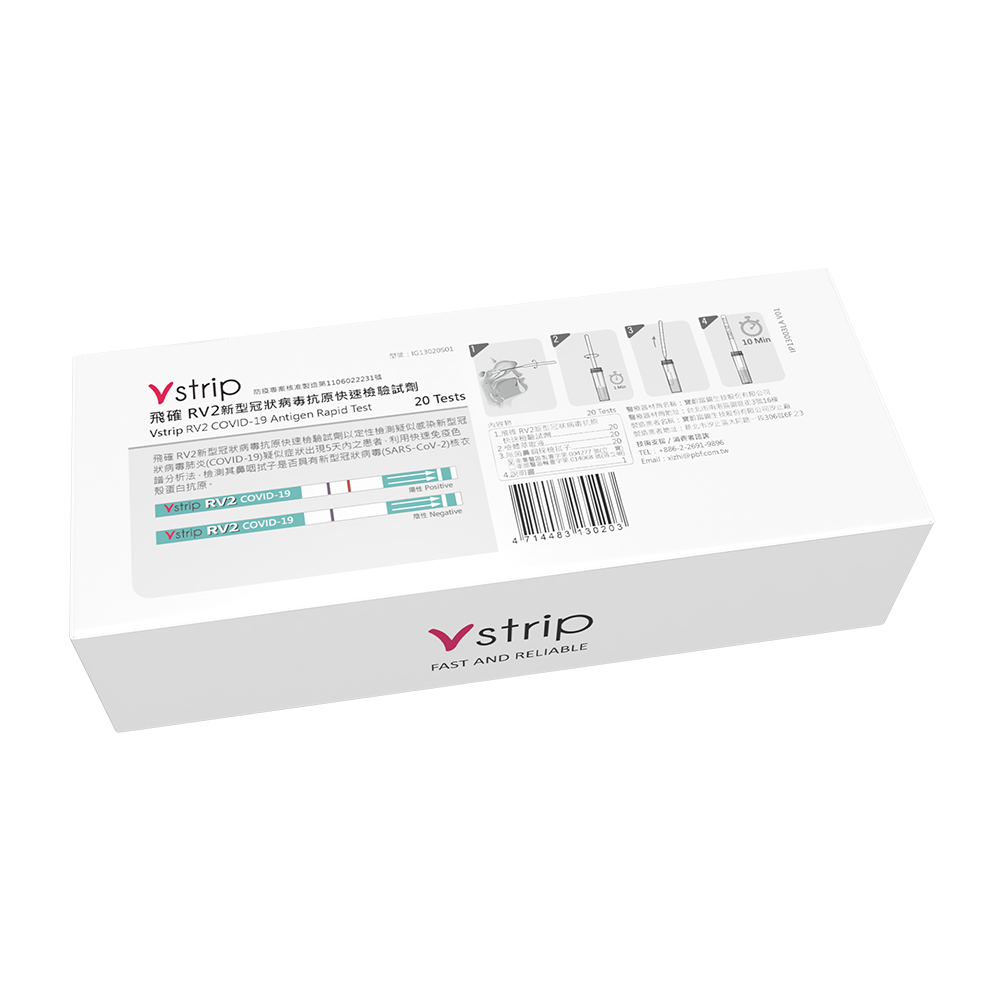
Product Features
Qualitative detection of patients suspected of being infected with the new coronavirus pneumonia (COVID-19) within 5 days of the onset of suspected symptoms, using rapid immunochromatographic analysis to detect whether their nasopharyngeal swabs contain the nucleocapsid of the new coronavirus (SARS-CoV-2) protein antigens.
The reaction time is 10-20 minutes.
Instructions
When collecting a nasopharyngeal specimen, use the nasopharyngeal swab provided in the kit.
To obtain correct test results, read the instruction manual carefully before testing and follow the instructions.
Additional Information
For in vitro diagnostic use only.
For emergency public health use only.
Epidemic Prevention Project Approval Manufacturing No. 1106022231.
Product model: IG13020S01.